4 Things to Know About China’s Vaccine Scandal
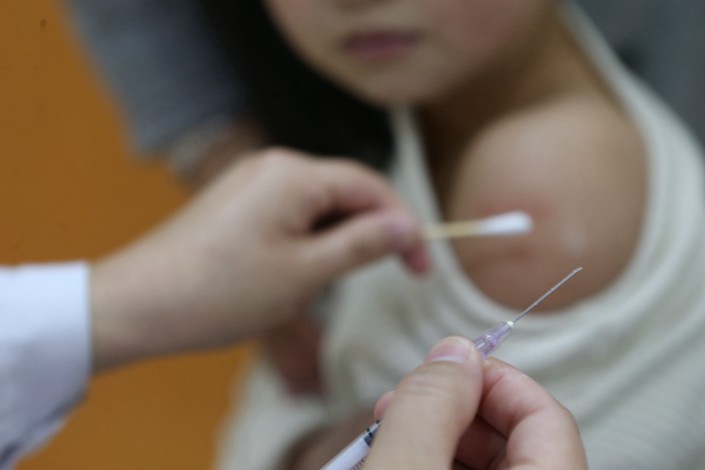
* Only 5% of vaccine batches are checked for effectiveness to avoid delaying the approval process and eating into the time before each batch’s expiration date
* Substandard vaccines are legally considered “inferior drugs,” not “counterfeit drugs,” and producing inferior drugs that have not been proved to seriously harm human health is not in itself enough justification for imprisonment under Chinese law
(Beijing) — The discovery that hundreds of thousands of substandard vaccines had been administered to children in China this week has sparked outrage, with President Xi Jinping calling the behavior of Changchun Changsheng Life Sciences, the company at the center of the scandal, “abominable.”
Changchun Changsheng, which officials say sold 250,000 doses of the mandatory diphtheria, pertussis and tetanus (DPT) vaccine to hospitals and clinics, mostly in Shandong province, has also been accused of tampering with production data for its rabies vaccine.
How do most Chinese people receive their vaccines, and who has been affected by Changchun Changsheng’s substandard vaccines?
Officially registered children and those born in hospitals are given certain mandatory vaccines for free, including those against tuberculosis and hepatitis B. Other vaccines, such as those offering protection against chickenpox, pneumonia and rabies, are optional and paid for by those who choose to receive them.
The substandard DPT vaccines sold by Changchun Changsheng were administered to more than 210,000 in Shandong, officials said.
How did substandard vaccines make it past regulators onto the market?
All vaccines sold in China must be submitted to the National Institutes for Food and Drug Control for approval. The country passed a law in 2006 requiring that all vaccines go through “lot release,” meaning that each batch of a vaccine must be inspected before being released for distribution.
But while inspectors test each batch for safety to make sure they have not been contaminated by dangerous microbes or toxic substances, only 5% of batches are checked for effectiveness to avoid delaying the approval process and eating into the time before each batch’s expiration date. Inspectors also tend to focus on whether the production process was consistent, which may be why Changchun Changsheng tampered with its production data.
What’s going to happen to the manufacturers? Could Changchun Changsheng personnel face the death penalty?
The company has been ordered to stop production, and police in the city of Changchun announced on Monday that they have arrested the company’s chairwoman, Gao Junfang, along with three other executives.
While some infuriated parents may be howling for blood, it’s possible that the vaccine manufacturers may not be punished as seriously as people executed in 2009 for their involvement in the tainted infant-formula scandal.
The substandard vaccines are legally considered “inferior drugs,” not “counterfeit drugs.” Someone caught manufacturing counterfeit drugs would face a standard sentence of three years in prison, but producing inferior drugs that have not been proved to seriously harm human health is not in itself enough justification for imprisonment under Chinese law.
However, if a manufacturer makes more than 50,000 yuan ($7,380) off the sale of substandard drugs, they could face sentences ranging from two years to life imprisonment, depending on the sales value of the drugs sold.
It also remains to be seen if prosecutors will consider substandard rabies vaccines to be dangerous to human health since inadequate protection against rabies could be deadly, even if the vaccine itself is harmless.
What does this scandal mean for China’s vaccine industry?
China’s human vaccine industry has been growing in recent years and is expected to reach a market size of as much as 31.2 billion yuan by 2021, thanks in part to the country’s loosened birth policy, according to research company Research and Markets. The country’s vaccine manufacturers have also been eyeing expansion into global markets, with Chengdu Institute of Biological Products becoming the first Chinese company to gain a “pre-qualification” from the World Health Organization for one of its vaccines in 2013.
But the industry has repeatedly been rocked by scandal, including a recent 2016 case that saw 130 people arrested for distributing 260 million yuan worth of vaccines without proper refrigeration, as well as the deaths of eight infants in 2013 after receiving hepatitis B vaccines produced by a Shenzhen-based manufacturer.
Investors responded to the latest scandal with concern, with dozens of vaccine and biotech stocks falling on Tuesday, and Changchun Changsheng’s own stock falling by the 10% daily limit for seven consecutive trading days.
Contact reporter Teng Jing Xuan (jingxuanteng@caixin.com)

- 1Cover Story: China Carves Out a Narrow Path for Offshore Asset Tokenization
- 2Drownings Shake Chinese Enthusiasm for Travel to Russia
- 3Over Half of China’s Provinces Cut Revenue Targets
- 4Li Ka-Shing’s Port Empire Hit by Forced Takeover Amid Panama Legal Dispute
- 5In Depth: China’s Mutual Fund Industry Faces Overhaul After a Banner 2025
- 1Power To The People: Pintec Serves A Booming Consumer Class
- 2Largest hotel group in Europe accepts UnionPay
- 3UnionPay mobile QuickPass debuts in Hong Kong
- 4UnionPay International launches premium catering privilege U Dining Collection
- 5UnionPay International’s U Plan has covered over 1600 stores overseas




