Analysis: The Closing Window for China’s mRNA Vaccine Developers
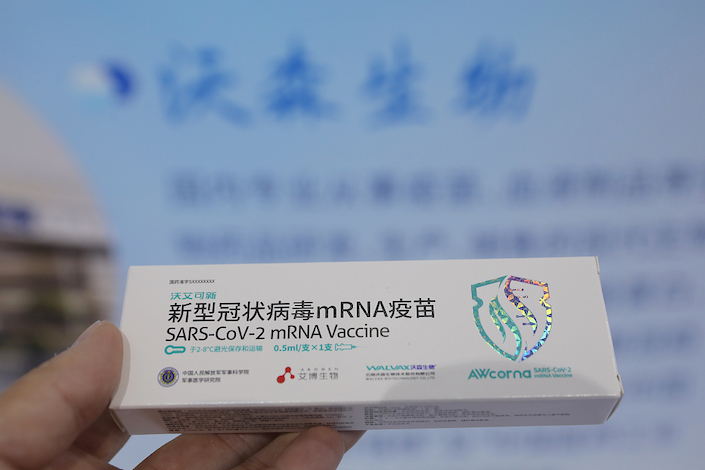
(Blue View) — Indonesia’s September emergency approval of an mRNA Covid vaccine developed by China’s Abogen Biosciences and Walvax Biotech was a milestone. But domestic clearance for such a shot is still lacking as the pandemic wanes worldwide, creating uncertainties for the product’s business outlook.
Over the past two years, the success of mRNA vaccines developed by BioNTech and Moderna in the global fight against the Covid-19 pandemic led to a boom for the new vaccine technology and attracted a continuous stream of investment.

- PODCAST
- MOST POPULAR






