Another Chinese Coronavirus Vaccine Candidate Enters Phase-Two Human Trials
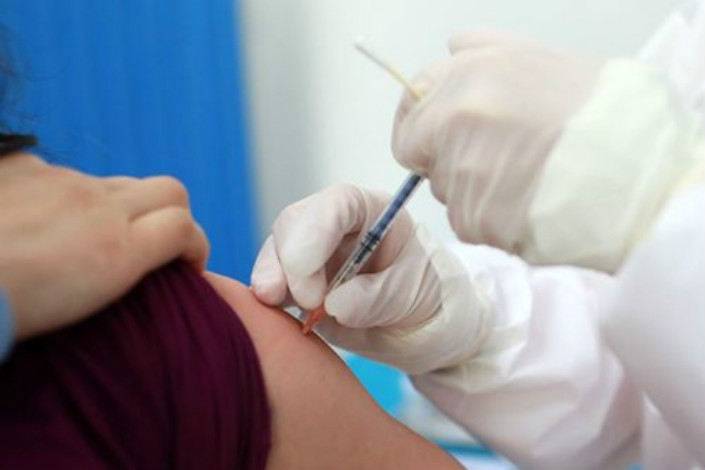
Another novel coronavirus vaccine candidate developed by Chinese scientists has started phase-two human trials as drugmakers worldwide race to find a way to stop the spread of the disease.
An inactivated vaccine developed by China National Pharmaceutical Group Corp. (Sinopharm) has entered the second phase of human trials, the company announced (link in Chinese) on social media on Friday. The progress comes only 12 days after it launched phase-one trials.
This is the second Chinese vaccine candidate to enter phase-two human trials after a potential adenovirus vector vaccine, jointly developed by Hong Kong-listed CanSino Biologics Inc. and the Institute of Biotechnology of the Academy of Military Medical Sciences, also started such trials on April 12.
 |
“Phase-two clinical trials are very critical, as we can see whether antibodies are produced after vaccines are injected, and we can tell the effectiveness based on the situation of the antibodies,” a vaccine developer told Caixin.
As of Thursday, Sinopharm had completed the first phase of trials, involving 96 people in three age groups in Wuzhi county, Henan province, and safety observations are ongoing, the company said.
Sinopharm is trialing inactivated vaccines, meaning that they are made from microorganisms that have been killed through physical or chemical processes.
Human clinical trials are divided into several stages. The first stage mainly evaluates safety, while the second stage evaluates both safety and the vaccination procedure, and the third phase focuses on effectiveness as well as safety. Each stage generally involves a progressively larger number of people.
A previous plan showed that Sinopharm planned to recruit a total of 1,456 volunteers who have never contracted the virus for the first two phases. The team told Caixin that the clinical tests will be carried out group by group. For instance, the trials will be expanded to elderly and younger people only after confirming the vaccine is safe for middle-aged people. The doses will also increase gradually.
“This design can push up the speed while ensuring high standards, quality and safety,” the research team said.
However, there is still a long way to go. Wang Junzhi, an academician at the Chinese Academy of Engineering, has said that it’s easier to recruit phase one and two volunteers, but the third phase is really when one can confirm the vaccine’s effectiveness and it usually needs to involve thousands of volunteers.
Given the ongoing pandemic, it might take more than six months to assess how much of an immune response the vaccine generates and an even longer time to confirm its protective immunity, Sinopharm said, adding that it might take a year to complete the third phase of clinical trials.
Denise Jia also contributed to this report.
Contact reporter Timmy Shen (hongmingshen@caixin.com, Twitter: @timmyhmshen) and editor Joshua Dummer (joshuadummer@caixin.com)
Caixin Global has launched Caixin CEIC Mobile, the mobile-only version of its world-class macroeconomic data platform.
If you’re using the Caixin app, please click here. If you haven’t downloaded the app, please click here.








