China Begins First Virus Antibody Trials on Healthy People
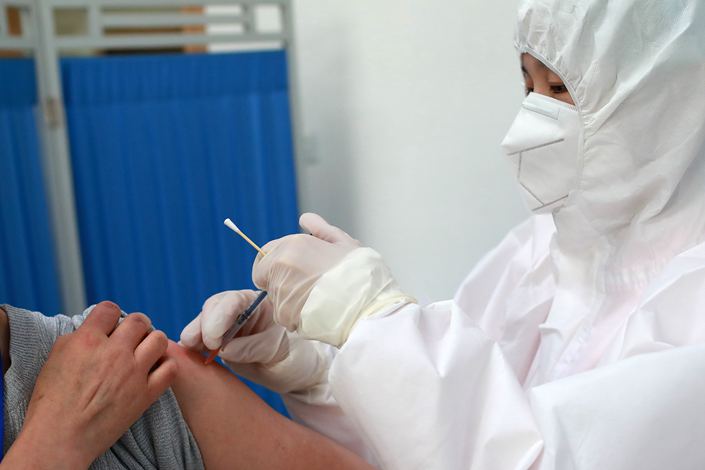
China has begun the world’s first trials of coronavirus-neutralizing antibodies on healthy people, marking a new step in the development of a treatment for Covid-19.
The National Medical Products Administration on Sunday gave the green light to Shanghai Junshi Biosciences Co. Ltd. and the Chinese Academy of Science’s Institute of Microbiology to start phase 1 clinical trials aimed at establishing whether the therapy is safe. The first group of participants have been administered the antibodies.
Zhang Wenhong, the high-profile infectious diseases department chief at Shanghai’s Huashan Hospital who is co-monitoring the study, said in a Junshi press release that researchers hoped the trial would provide supporting data for further clinical plans. “Neutralizing antibody therapies will hopefully lead the way in becoming treatment choices for fighting the coronavirus,” he said.
Junshi’s shares in Hong Kong rose by 20% at the start of Monday trading as investors responded to the news. The pharmaceutical company ended the day up 7.5% at HK$46.75 ($6.03).
Neutralizing antibodies are specific proteins that bind to things like viruses and bacteria to stop them from working. Our immune systems produce them naturally to fight disease. As part of the fight against the coronavirus, scientists are exploring how to identify and reproduce neutralizing antibodies and administer them to help people recover from Covid-19 and prevent healthy people from getting infected.
Researchers injected volunteers with antibodies cloned from people who previously had SARS-Cov-2, the virus that causes Covid-19. The antibodies attach to spiky proteins on the surface of the virus and stop it from joining to the receptors that allow it to enter human cells, thereby preventing infection.
Junshi has not disclosed some details of the trials. The company told Caixin that it will release further information at a “suitable time.”
The randomized, double-blind, placebo-controlled trial is overseen by Zhang and his colleague Zhang Jing, who heads the hospital’s antibody research center.
If the researchers conclude that the treatment is safe, they will likely proceed to phase 2 trials designed to test whether it actually works. From there, the therapy would have to pass a further stage of testing before it could be widely administered.
The Chinese scientists are reportedly the first to test a neutralizing antibody treatment on healthy individuals. Last week, U.S. pharmaceutical company Eli Lilly and Co. Ltd. said it had collaborated with Canada’s AbCellera Biologics Inc. to start phase 1 trials of a similar therapy previously used to treat sick Covid-19 patients.
Antibody therapies differ from vaccinations, which typically involve injecting people with weak or inactive forms of viruses or bacteria to prime the body’s immune response.
Scientists worldwide are racing to develop coronavirus vaccines. Last week, governments and organizations pledged $8.8 billion to Gavi, the Gates Foundation-backed global vaccine alliance, in order to fund Covid-19 immunizations.
The pandemic has infected 7.1 million people and killed 406,000 globally, according to a tally kept by Johns Hopkins University that uses official government data.
Contact reporter Matthew Walsh (matthewwalsh@caixin.com) and editor Joshua Dummer (joshuadummer@caixin.com)

- PODCAST
- MOST POPULAR






