Sinovac Demonstrates Safety of Vaccine that Doesn’t Need Deep Freeze
Sinovac Biotech Ltd. researchers have published data from early trials showing their vaccine is safe for healthy people to take, and that it sparks an immune response, though the company has yet to determine if it stops people from catching Covid-19.
Sinovac tested the vaccine candidate, known as “CoronaVac,” on about 750 healthy adults up to the age of 59, according to the combined phase 1 and 2 trial results published in Lancet Infectious Diseases on Tuesday.
Those who had the jab were found to produce antibodies to the virus that causes Covid-19 — though at lower levels than those in people who have been infected and recovered from the wild virus.
That was lower than antibodies from vaccine trials by world leaders Pfizer Inc. and Moderna Inc., which are using a different vaccine technology, and which recorded antibody levels in patients that were about the same as those who had contracted the virus.
However, the researchers said they still expect CoronaVac to protect against Covid-19, citing their experience with other vaccines and evidence gathered from testing it on monkeys.
Early phase trials generally check if the vaccine is safe and provokes an immune response, and are used to determine things like dosing and side effects. Most of the side effects recorded in the early CoronaVac trials were mild, the researchers said.
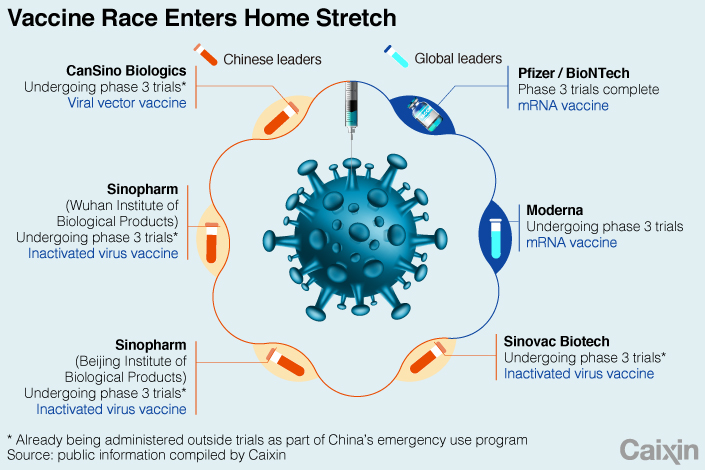 |
Whether CoronaVac actually prevents people catching the coronavirus is currently being assessed in phase 3 studies in Brazil, Indonesia and Turkey, along with the closely watched T-cell response triggered by the vaccine.
The randomized, double-blinded controlled trials — considered a gold standard by scientists as it lets them exclude coincidental reasons why a drug may appear to succeed or fail — were carried out in the town of Suining, East China’s Jiangsu province.
Gang Zeng, a Sinovac researcher involved in the study, acknowledged that many vaccine candidates and technologies are being explored, but said CoronaVac could be an “attractive option” because it can be stored in a standard refrigerator between 2 and 8 degrees Celsius.
That could be a nod to revelations that the Pfizer vaccine, which is based on leading-edge technology and has already completed trials, needs to be kept at around minus 70 degrees Celsius, which could make storing and transporting it difficult, particularly in developing countries.
In a further potential benefit, Sinovac researchers believe their vaccine may be able to be stored for up to three years. “However, data from phase 3 studies will be crucial before any recommendations about the potential uses of CoronaVac can be made,” Zeng said.
The trials took place between April 16 and May 5.
Chinese health authorities have already inoculated hundreds of thousands of people in China with experimental coronavirus vaccines outside of clinical trials, including CoronaVac, under an emergency use program that began on July 22 and was officially revealed in August.
About 90% of Sinovac staff and their families have already taken CoronaVac under emergency use provisions, the company’s CEO Yin Weidong revealed in early September.
The program, which initially focused on at-risk groups such as health workers and border personnel, has since been expanded.
Contact reporter Flynn Murphy (flynnmurphy@caixin.com) and editor Michael Bellart (michaelbellart@caixin.com)
Download our app to receive breaking news alerts and read the news on the go.

- PODCAST
- MOST POPULAR






