Chinese Co-Developer of DNA Covid Vaccine Raises $66 Million
A Chinese vaccine-maker that is developing a DNA coronavirus vaccine with U.S.-based Inovio Pharmaceuticals Inc. has raised $66 million in new funding, according to a company press release issued late last month.
Beijing Advaccine Biotechnology Co. Ltd. secured the strategic investment in its latest financing round, which was led by Matrix Partners China Management Ltd. and Hony Capital Ltd.
The investment came as Advaccine’s INO-4800 coronavirus vaccine candidate is in the midst of phase 2 clinical trials, said Liu Xiaoyan, strategic director of Advaccine. The company expects the DNA vaccine candidate to enter phase 3 trials in the second quarter.
Liu added that the vaccine could be put into use by the end of this year if all goes to plan.
Founded in 2009 with a registered capital of nearly 30 million yuan ($4.5 million), Advaccine primarily develops vaccines, including a respiratory syncytial virus vaccine and hepatitis B vaccine, though none of its inoculations has made it to the market as yet.
In January 2020, Advaccine partnered with Nasdaq-listed vaccine manufacturer Inovio to work on the INO-4800 DNA vaccine candidate.
Read more
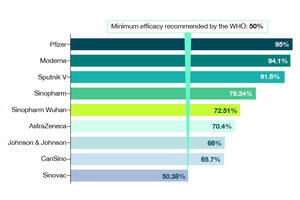
Tip Sheet: Key Facts to Know About China’s Vaccine Race
Compared with an mRNA vaccine, a DNA vaccine is easier to produce, store and ship, according to the company. Wang Bin, founder and chairman of Advaccine, has said that DNA vaccines can be quickly and reliably produced, and can be stored for five years at 2 C to 8 C, or one year at room temperature. By comparison, Pfizer Inc. and BioNTech SE’s mRNA Covid-19 vaccine needs to be stored at -70 C, and Moderna Inc.’s mRNA vaccine has to be stored at -20 C.
Under Advaccine’s January agreement with Inovio, it has the right to market the DNA vaccine in China.
In September, Advaccine said it had acquired (link in Chinese) Si Ao Biotechnologies Suzhou Co. Ltd., which owns a nearly 20,000-square-meter vaccine production facility in Suzhou, East China’s Jiangsu province.
Liu said that the company will be able to produce 20 million doses a year once the DNA vaccine hits market, and the annual capacity could grow to 100 million doses within about half a year.
Contact reporter Timmy Shen (hongmingshen@caixin.com) and editor Michael Bellart (michaelbellart@caixin.com)
Download our app to receive breaking news alerts and read the news on the go.
Follow the Chinese markets in real time with Caixin Global’s new stock database.